
SC.912.L. Properties of Water
Discuss the special properties of water that contribute to Earth's suitability as an environment for life:
1. Cohesion and Adhesion
2. Ability to Moderate Temperature
3. Expansion Upon Freezing
4. Versatility as a Solvent
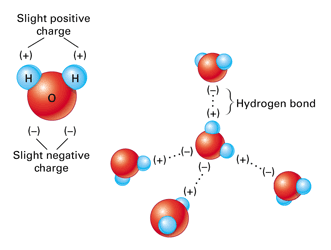
Polarity and Hydrogen Bonding
Water is a polar molecule. Polar molecules have slightly charged regions. The molecule is a bit like a magnet with two poles.
A hydrogen bond is a weak bond formed between slightly positive hydrogen atoms and slightly negative oxygen atoms.
1. Cohesion and Adhesion
The attraction between molecules of the same substance is called cohesion. The hydrogen bonds in water cause water molecules to stick together. Because a single water molecule can be involved in as many a four hydrogen bonds at the same time water molecules are extremely cohesive. Cohesion causes water molecules to be drawn together, which is why drops of water form beads on a smooth surface.
What does it mean for life?
Cohesion allows for small droplets of water to form larger bodies of water making the water less vulnerable to evaporation and more available to organisms
Because of cohesion, water molecules remain joined together as they move within or between the cells.
Cohesion also produces surface tension, explaining why some insects and spiders can walk on a pond's surface. The insects are light enough so that they do not break the hydrogen bonds holding the water molecules together

The attraction between molecules of different substances is called adhesion. In other words, adhesion is the tendency of surface molecules to stick to other surfaces. Water has strong adhesion
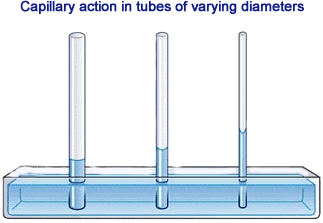
You see adhesion when you add water to a graduated cylinder. The water dips slightly in the center. This is because the adhesion between water molecules and the glass is stronger than the cohesion between water molecules

Adhesion causes water to rise in a narrow tube against the force of gravity. This effect is called capillary action.
What does it mean for life?
Water fills the internal transport systems of plants and animals. Adhesion causes water to stick to the sides of blood vessels and to the vascular tubes in plants. Cohesion causes water molecules to remain joined together as they move.
Because of these cohesive and adhesive properties, water allows for the efficient transport of nutrients and wastes within an organism.
Adhesion is one of the forces that pulls water up the roots and stems of plants, toward the leaves where photosynthesis occur.
Because of cohesion and adhesion water flows upward against the force of gravity through the stem of a plant.

2. Ability to Moderate Temperature / High Heat Capacity
Water acts as a regulator of temperature. This works at a large scale for the entire planet as well as at smaller scales. Water is able to effectively mitigate drastic changes in temperature due to its high heat capacity.
The many hydrogen bonds between water molecules cause water to have a relatively high heat capacity. A high heat capacity means that more heat energy is required to raise the temperature of water than many other substances, such as air or sand.
It is hard to get water to change temperature, either up or down. This is due to the hydrogen bonding. The bonds must be broken or formed first before any temperature changes will be seen. When you add heat energy to water it will first go toward breaking up the hydrogen bonds.

This allows bodies of water such as oceans and lakes to absorb large amounts of heat with only small changes in temperature.
What does it mean for life?
The organisms living in the water are protected from drastic temperatures changes.
Organisms typically cannot handle drastic, rapid changes in temperature very well and the high heat capacity of water helps control how fast temperatures in living organisms can change.
The water's high heat capacity helps keep aquatic environments at fairly stable temperatures.
Since life is based on water and the temperature of water rises and falls slowly living organisms are able to maintain normal internal temperatures and are protected from rapid temperatures changes (help maintain homeostasis)
The presence of vast oceans and water vapor in the atmosphere, both of which retain and circulate energy. By capturing and moving energy around the world by currents, the water in the ocean and in the atmosphere both help the Earth warm more evenly
Bodies of water store heat during the day and release it at night.
Example: In places that get very little rainfall, such as the desert in Arizona, daytime temperatures can get very high and nighttime temperatures can get very low, often dropping dramatically as soon as the Sun sets. This happens because there is not enough water to remove the heat from the atmosphere during daytime and to release it at night.
In Florida, daytime and nighttime temperatures usually only differ by 5 to 10°C, while temperatures in the desert often differ by as much as 40°C. Water plays an important role in keeping temperatures in Florida from fluctuating dramatically. Bodies of water store heat during the day (remove heat from the atmosphere) and release it at night.

3. Expansion upon freezing
The density of water is 1 g/cm³ and the density of ice is 0.94g/cm³. Ice is less dense than liquid water. Because of that density difference, the ice floats.
When water freezes, the molecules separate a little, stabilize hydrogen bonds, form a 3D lattice, and make the water much less dense.

The lower density of water in the solid state than the liquid state allows ice to form at the surface of aquatic habitats, leaving a liquid environment beneath.
So what does this mean for life?
The layer of ice acts as an insulator and prevents the deeper water from freezing solid, because of this aquatic life survives the winter.
Since ice floats, organisms are able to survive underwater in the icy Polar Regions and even on top of the ice since thick ice is strong.
Polar bears use the floating ice as a platform for hunting
Floating ice can melt because it’s exposed to the Sun and warm surface temperatures. Any water that freezes can be thawed and vice versa.
Ice also reflects a lot of heat and light away from the Earth.
If ice DIDN’T float.
First, a lot more sunlight would be absorbed by the Earth. This would result in a warmer planet.
Secondly, if ice sank to the bottom of Earth’s oceans, lakes, and rivers, it would stay cold and, over time, build up.

4. Versatility as a solvent
Water's polarity and bonding make it a powerful solvent. A solvent is substance in which other substances dissolve to form a solution. Water can dissolve many different compounds. In fact water's ability to dissolve so many different substances has earned it the nickname of the "universal solvent".
Water’s polarity gives it the ability to dissolve both ionic compounds and other polar molecules. Water easily dissolves salts, sugars, minerals, gases, and even other solvents such as alcohol.
A Salt Solution
When an ionic compound such as sodium chloride is placed in water. water molecules surround and separate the positive and negative ions.

What does this mean for life?
Living things are composed mostly of water. Water accounts for approximately 60 percent of the mass of the human body. As a result, the chemical reactions that take place within living things do so in a water environment. Nearly everything that cells do—from growth and development to movement—takes place by means of chemical reactions in a water environment.
Water's ability to dissolve many substances enables it to deliver essential nutrients to cells in plants, animals and other organisms
This solutions of ions and molecules dissolved in water allow chemical reactions to occur much more frequently, which is an important feature to allow organisms to respond to their environment, growth and development.
Many substances needed by cells enter the cell while dissolved in water.
Many of the chemical reactions take place in a water-based environment.
